Clinical trials involve testing new drugs and medical equipment on human subjects. In clinical trials, patients get access to medicines and equipment long before they are on the market.
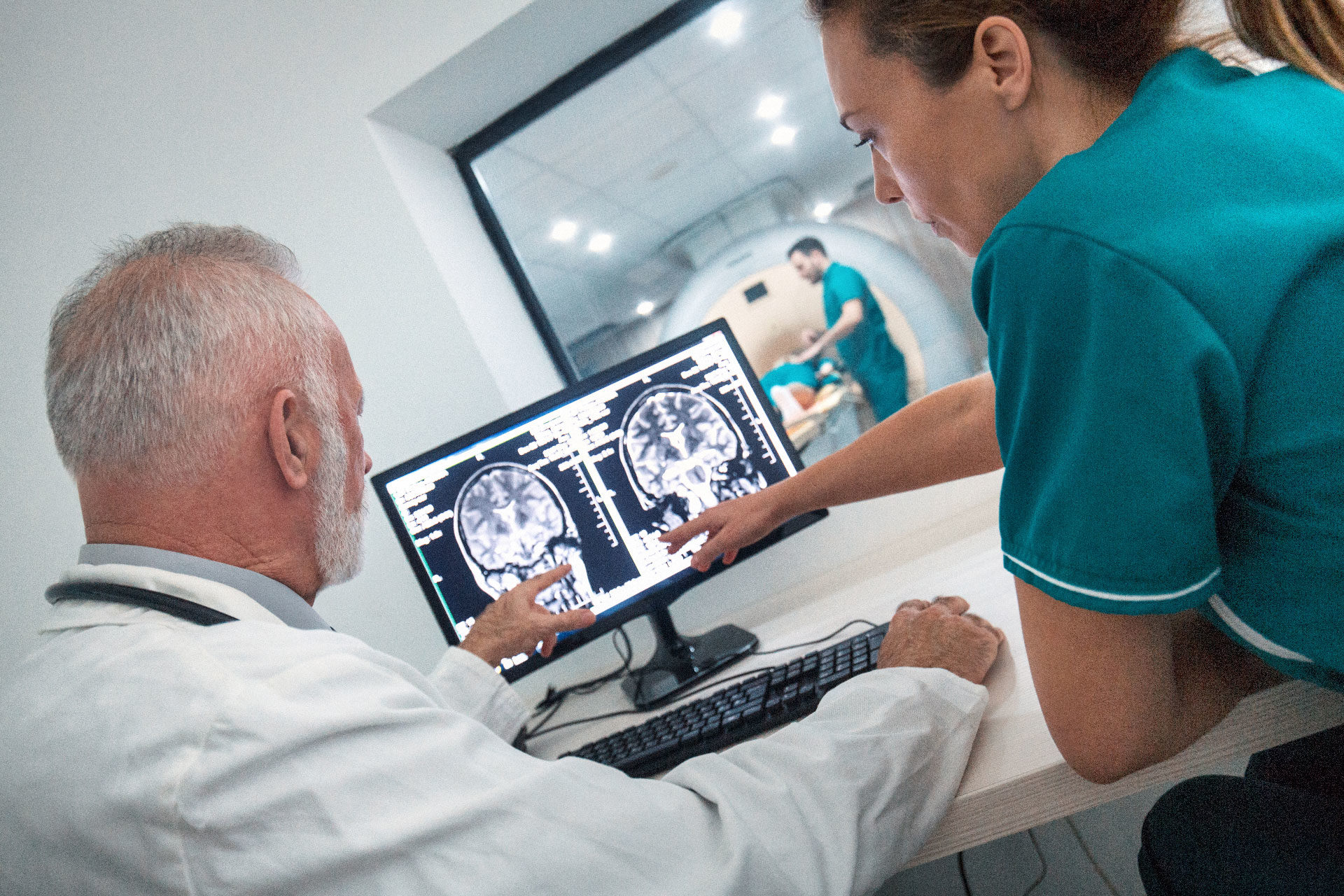
‘Clinical trials are the key to medical development. On average, patients who participate in clinical trials get access to experimental treatment six to seven years before the treatment is available on the market,’ says Siri Kolle. Kolle is Vice President Clinical in Inven2 and is responsible for Inven2’s clinical trials.
Clinical trials give health personnel important experience with new drugs and treatment methods, research experience and access to international networks.
‘It is also important for the Norwegian health industry to be able to test their treatments locally, and Norwegian innovations will then benefit patients in Norway at an early stage,’ says Kolle.
A well-organised system
Inven2 manages study agreements with the industry on behalf of all hospitals in the South-Eastern Norway Regional Health Authority as well as the University Hospital of Northern Norway (UNN) and Nordland Hospital Trust. Inven2 is also responsible for following up the financial aspects of the agreements.
‘This is a good, well-organised system and it ensures an arms-length distance between the industry and the hospital, which in turn ensures transparent collaboration,’ says Kolle.
Inven2 helps to simplify the process of starting trials in Norway, and makes continuous efforts to streamline the process of putting agreements in place in order to ensure that trials can start as quick as possible.
‘The fact that we can have a fast start up process in Norway is an important competitive advantage for us. There is strong global competition to attract clinical trials, so we’ve got to be competitive,’ says Kolle.

CONTACT
Siri Kolle, M.Sc
Vice President Clinical
siri.kolle@inven2.com
Mob:(+47) 41 51 37 46



 Norsk
Norsk

