


I 2017 har vi sett sterk vekst i verdien av innovasjon og omgjøring av forskning til samfunnsnyttige tjenester og produkter.
Mange av selskapene vi har vært med på å etablere, utvikler seg mer enn tilfredsstillende. Hele selskapsporteføljen er ved årsskiftet verdt over ni milliarder kroner. Dette tilsvarer en tredobling av totalverdien på tre år. Det er en voldsom vekst på kort tid i kroner og øre, men viser også at de produktene som blir utviklet på basis av forskning ved Universitetet i Oslo og helseforetakene i Helse Sør-Øst dekker et behov i samfunnet.
Les mer
Nye ideér
nye selskaper etablert
nye lisensavtaler
nye patentsøknader
nye kliniske studier
pågående kliniske studier
milliarder kroner i samlet verdi på portefølje-selskaper
millioner kroner til ny forskning
millioner kroner innhentet i privat kapital
Vi gjør en forskjell ved å omgjøre forskning og kunnskap til samfunnsnyttige produkter og tjenester.

Forskning og kunnskap er nøkkelen til å løse globale samfunnsutfordringer innen helse, klima og miljø. Dyktige forskere og klinikere fra halve Norge melder inn idéer og oppdagelser til oss. Vi utvikler og forvalter idéene, og går videre med idéer vi kan omgjøre til samfunnsnyttige produkter og tjenester.
Vi har startet bedrifter som utvikler bedre kreftbehandling, bekjemper antibiotikaresistens og identifiserer sikkerhetsbrister i komplekse IT-systemer. To tredjedeler av våre bedrifter og lisenser er innen livsvitenskap, da vårt økosystem er spesielt sterkt innen dette fagfeltet.
En annen viktig oppgave vi har, er å forvalte avtaler om kliniske studier på vegne av sykehusene i vår region. Vi jobber for at flere internasjonale selskaper skal legge sine kliniske studier til Norge. Slik kan mer nyskapende behandling komme flere pasienter i Norge til gode.
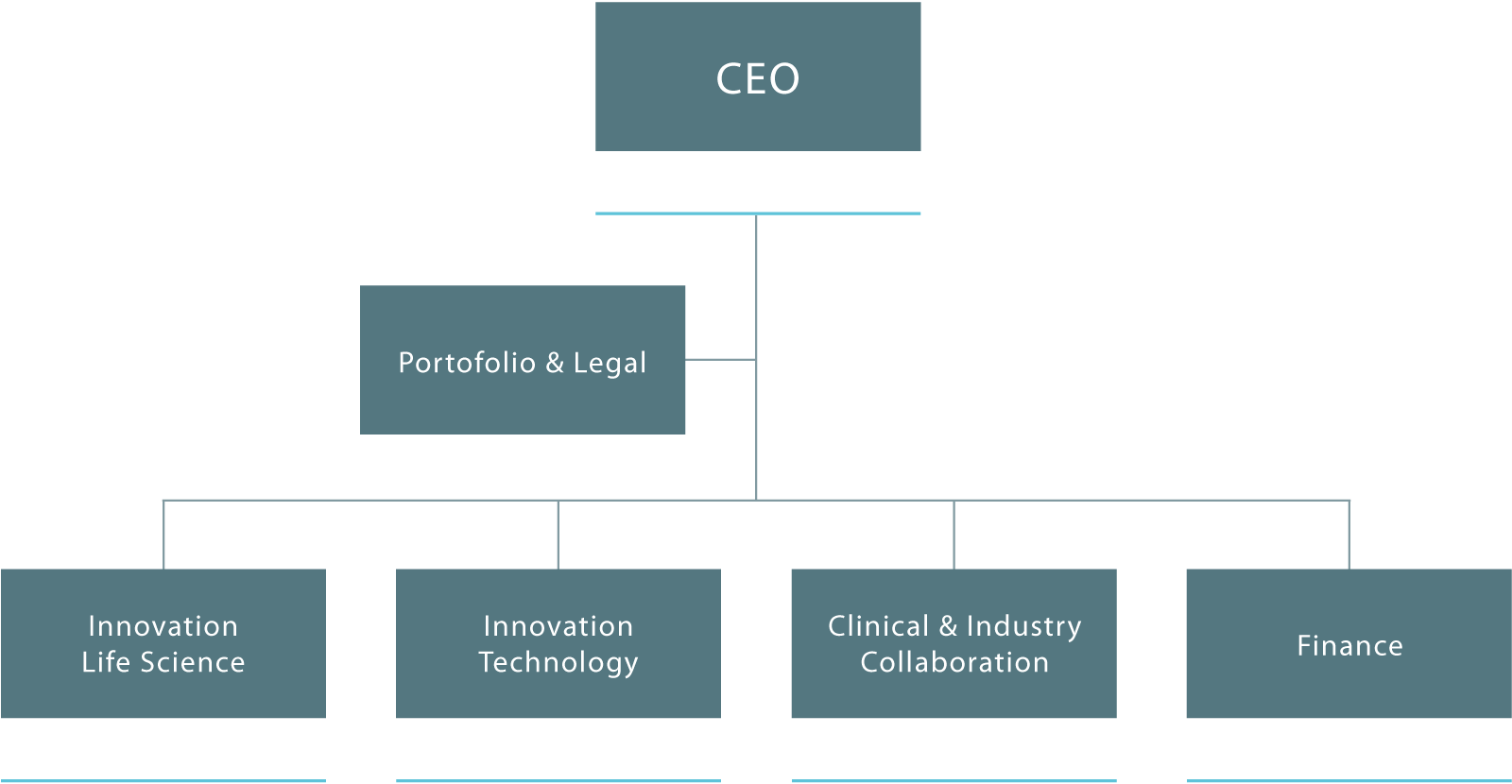
Inven2 er et aksjeselskap eid av Universitetet i Oslo og Oslo universitetssykehus. Selskapet har 34 ansatte.
2017 var et spennende år for Inven2 og våre porteføljeselskaper. Se et utvalg av suksesshistorier fra året som gikk.

Kliniske studier er utprøving av nye medisiner og medisinsk utstyr for behandling på mennesker. Studier kan gjøres på både friske frivillige og pasienter. Kliniske studier gir helsepersonell viktig erfaring med nye legemidler og behandlingsmetodikk, forskningserfaring, samt internasjonalt nettverk.
Les mer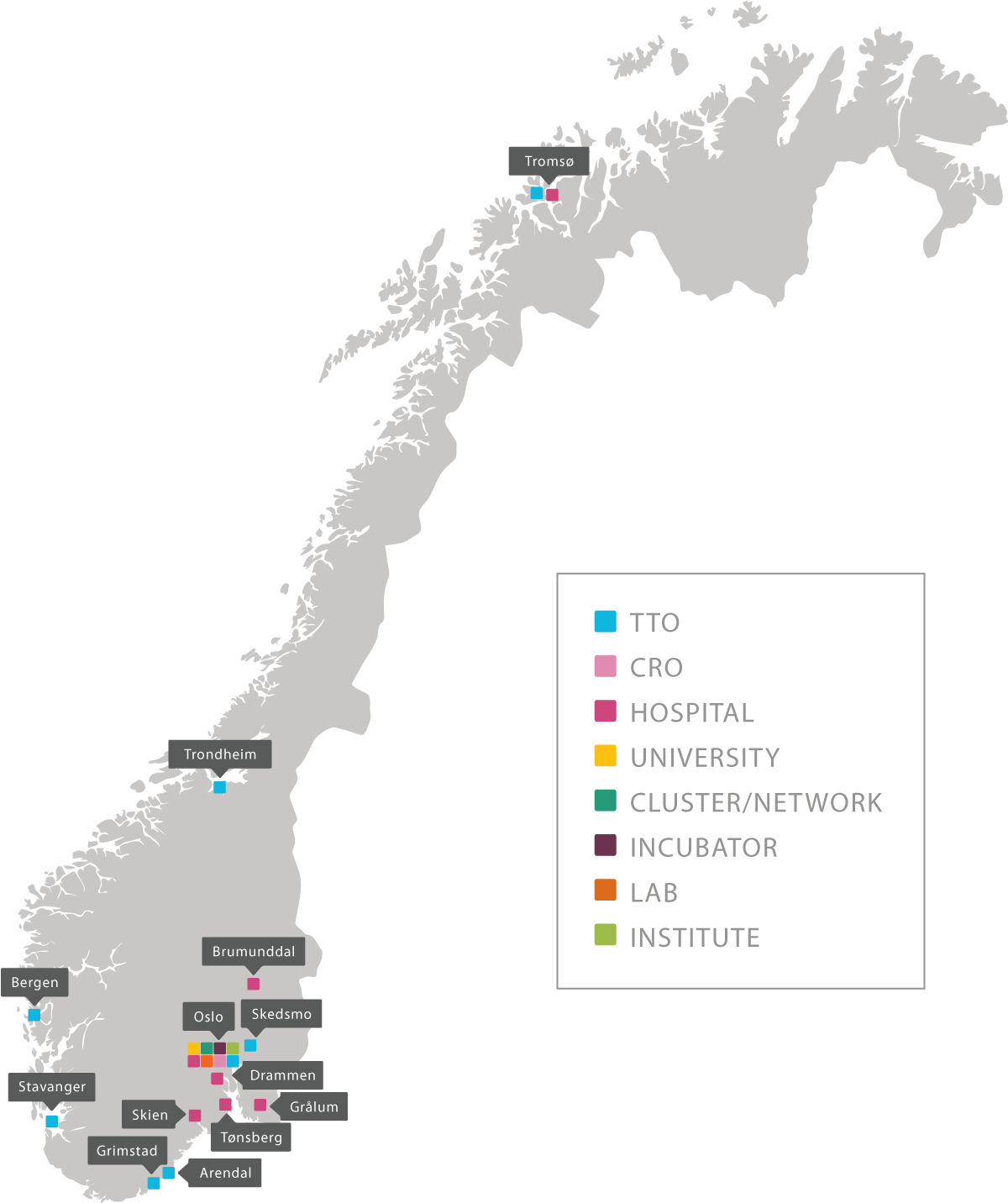
Inven2 er en del av et vitalt og stadig større økosystem innen forskning og utvikling.
I 2017 har dette miljøet blitt styrket med inkubatoren ShareLab og Catapult Life Sciences.
Les mer
Inven2s årlige idékonkurranse hvor vi kårer beste idé til et produkt eller tjeneste
I 2017 kom det inn hele 64 idéer. Det var mange gode bidrag, men juryen klarte til slutt å kåre tre finalister som hver fikk 25.000 kroner til å videreutvikle sin idé. Finalistene var «Min Pilot – en app for bedre samhandling om psykisk helse», «3D tool to plan and assist operations» og «Målrettet behandling av alvorlige hjerterytmeforstyrrelser».
Sistnevnte stakk av med seieren etter en intens avstemning på sosiale medier, og vant dermed hovedpremien på totalt 250.000 kroner.
Les mer om vinneren
Vi er stolt medarrangør av Cutting Edge-festivalen, Norges største vitenskaps- og teknologievent, hvor vi sammen med våre partnere Oslotech og Universitetet i Oslo viser frem det siste innen vitenskap, teknologi og innovasjon.
www.cuttingedgefestival.no Se filmen fra Cutting EdgeBesøkende
Foredrag
Workshops
Debatter
1-1 møter
Utstillere

Over 150 møter mellom forskere, etablerte bedrifter og start-ups ble gjennomført på matchmakingen under Cutting Edge 2017.
Les mer
Inven2 og studentorganisasjonen StartUiO samarbeider for å stimulere til gründerskap og nye bedrifter spunnet ut av idéer fra dyktige studenter. Resultatet av de målrettede aktivitetene er fire nye studentselskaper under etablering som skal delta i oppstartprogrammet Inven2Start.
Les merInven2 ønsker å gjøre en forskjell ved å omgjøre forskning og kunnskap til samfunnsnyttige produkter og tjenester. Siden oppstart har vi bidratt til at mange produkter og tjenester har kommet samfunnet til gode. Her er et utvalg av dem.
Inven2 omorganiserte deler av virksomheten i 2017, og har startet opp en egen satsing på digitalisering og e-helse. Formålet er å bidra til utviklingen av flere forskningsbaserte produkter og tjenester, og da særlig innen digital teknologi.
Inven2 håndterer samarbeids- og oppdragsforskningsavtaler med industri på vegne av helseforetakene i Helse SørØst og Univeristetsykehuset i Nord-Norge. Inven2 har kompetanse på avtalehåndtering, forhandling og økonomioppfølging av industrisponsede kliniske studier og andre former for industrisamarbeid. Vi fasiliterer og rådgir utprøvere med tanke på budsjett og oppsett av avtaler.
Meld ny studieI kort så tilbyr vi tjenester innen forretningsutvikling, patentering, kommunikasjon, lisensiering, bedriftsetablering og innhenting av finansiering. I tillegg har vi et stort nettverk av samarbeidspartnere nasjonalt og internasjonalt.
Meld ny idé