

nye ideér (DOFIer)
milliarder kroner i samlet verdi
på porteføljeselskaper
nye lisensavtaler
nye patentsøknader
nye kliniske studier
pågående kliniske studier
nye selskaper etablert
millioner kroner til ny forskning
milliarder kroner innhentet
i
privat kapital

– hvordan ta forskning til markedet?
Inven2 sitt kjerneområde er å ta resultatene fra forskning til et produkt eller en tjeneste i et marked. Det gjøres gjennom en verdikjede som starter med idéspeiding og markedsføring av Inven2 sine tjenester rettet mot forskere ved både Universitetet i Oslo sykehusene i Helse Sør-Øst.
Les merInven2 arrangerer en rekke aktiviteter hvert år for å stimulere til innovasjon. Noe gjør vi alene, men mye gjør vi sammen med aktører i det store økosystemet vi er en del av.

for innovasjon
Samarbeid mellom næringsliv og forskning er viktig for mer innovasjon, og fører til flere kommersialiseringer. Inven2 jobber målrettet med industrisamarbeid gjennom en rekke ulike prosjekter.
Les mer
Kliniske studier er utprøving av nye medisiner og medisinsk utstyr for behandling på mennesker. Studier kan gjøres på både friske frivillige og pasienter. Kliniske studier gir helsepersonell viktig erfaring med nye legemidler og behandlingsmetodikk, forskningserfaring, samt internasjonalt nettverk.
Les mer
Inven2 er landets største teknologioverføringsselskap, TTO, og det innebærer at vi har ansvaret for å vurdere og kommersialisere innovativ forskning fra Universitetet i Oslo, Oslo universitetssykehus og helseforetakene i Helse Sør-Øst. Dette er våre eiere.
I 2019 arbeidet jeg og mine 31 medarbeidere hardt for å ivareta dette ansvaret på en god måte, som vi har gjort nå i snart 10 år. Og 2019 er et år som bærer fruktene av dette arbeidet.
Les merVi gjør en forskjell ved å omgjøre forskning og kunnskap til samfunnsnyttige produkter og tjenester.

Forskning og kunnskap er nøkkelen til å løse globale samfunnsutfordringer innen helse, klima og miljø. Dyktige forskere og klinikere fra halve Norge melder inn idéer og oppdagelser til oss. Vi utvikler og forvalter idéene, og går videre med idéer vi kan omgjøre til samfunnsnyttige produkter og tjenester.
Vi har startet bedrifter som utvikler bedre kreftbehandling, bekjemper antibiotikaresistens og identifiserer sikkerhetsbrister i komplekse IT-systemer. To tredjedeler av våre bedrifter og lisenser er innen livsvitenskap, da vårt økosystem er spesielt sterkt innen dette fagfeltet.
En annen viktig oppgave vi har, er å forvalte avtaler om kliniske studier på vegne av sykehusene i vår region. Vi jobber for at flere nasjonale og internasjonale selskaper skal legge sine kliniske studier til Norge. Slik kan mer nyskapende behandling komme flere pasienter i Norge til gode.
Inven2 er et aksjeselskap eid av Universitetet i Oslo og Oslo universitetssykehus. Selskapet har 32 ansatte.

Vi er stolt medarrangør av Cutting Edge-festivalen, Norges største vitenskaps- og teknologievent, hvor vi sammen med våre partnere Oslotech, Universitetet i Oslo og Conventor viser frem det siste innen vitenskap, teknologi og innovasjon.
www.cuttingedgefestival.no Se filmen fra Cutting EdgeBesøkende
Foredrag
Workshops
Debatter
1-1 møter
Utstillere

Nesten 90 1:1 møter mellom forskere, etablerte bedrifter og oppstartsbedrifter ble gjennomført under Cutting Edge i år. «Vi fikk viktige tilbakemeldinger,» forteller forsker Henriette Andresen etter møte med Pfizer.
Les merInven2 har en portefølje bestående av 51 selskaper. I 2019 ble syv nye etablert, hvorav fire er studentselskaper og tre er selskaper som skal kommersialisere forskningsbaserte innovasjoner. Samlet har selskapene en verdi på 12,5 milliarder norske kroner. Selskapene hentet totalt 1,3 milliarder kroner i egenkapital for å utvikle seg videre i 2019. Samtidig ble 40 millioner kroner pløyd tilbake til forskning ved Universitetet i Oslo og Oslo universitetssykehus.
Verdiøkningen på porteføljen i 2019 var på hele 29 prosent, som blant annet skyldes børsnoteringen til kreftselskapet Ultimovacs ASA og verdiøkningen til Vaccibody AS.
Vaccibody utvikler persontilpassede kreftvaksiner og presenterte i november lovende kliniske data.
De er i dag det høyest prisede bioteknologiselskapet i Norge med en verdi i gråmarkedet på 4,9 milliarder kroner.
Ultimovacs sin børsnotering og kliniske utvikling av den universelle kreftvaksinen UV1 kan du lese mer om nedenfor. Et annet selskap du kan lese mer i dybden om er sikkerhetsselskapet Offpad. Selskapet står foran et mulig kommersielt gjennombrudd for sin sikkerhetsløsning for å hindre spionasje og hacking av mobil og PC til stats- og industriansatte på tjenestereiser. Det siste selskapet du kan lese mer om er Serca Pharmaceuticals. Målet deres å utvikle en innovativ behandling av pasienter rammet av hjerteinfarkt. I 2019 tok de viktige kommersielle skritt for å komme dit.
2019 var også året hvor Prophylix Pharma AS, der Inven2 har en eierandel, ble solgt til det amerikanske selskapet Rallybio. De utvikler legemidler til behandling av sjeldne sykdommer.
Inven2 ønsker å gjøre en forskjell ved å omgjøre forskning og kunnskap til samfunnsnyttige produkter og tjenester. Siden oppstart har vi bidratt til at mange produkter og tjenester har kommet samfunnet til gode. Her er et utvalg av dem.
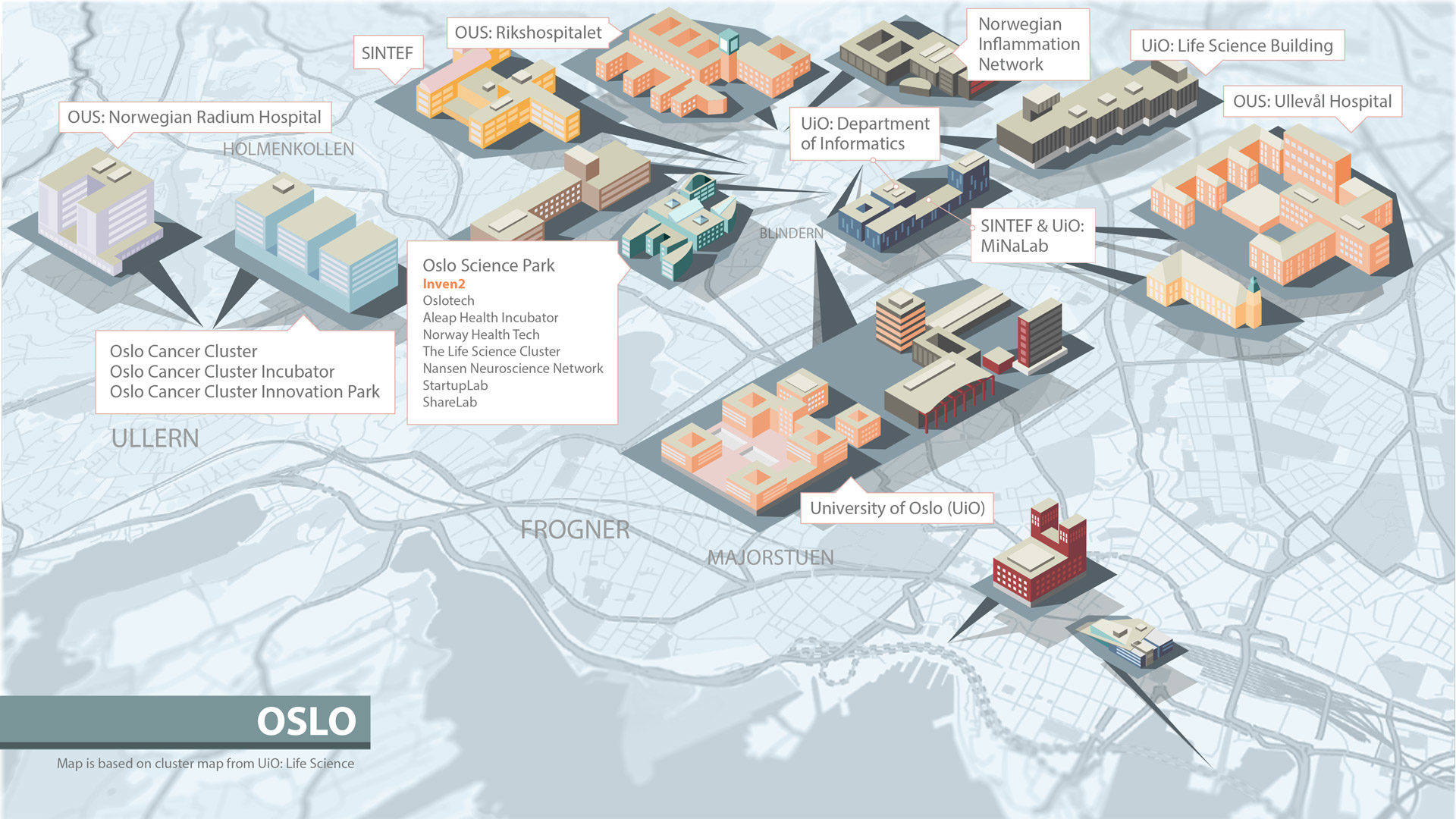
Inven2 er en del av et vitalt og stadig større økosystem innen forskning og utvikling. Bare i nærområdet til Inven2s lokaler i Forskningsparken finnes en rekke klynger og inkubatorer, samt Universitetet i Oslo og Oslo universitetssykehus.
Les mer